
Axis Spine Technologies raises $12.5m after successful product pre-launch


London, UK – March 25, 2022 – Seymourpowell, a leading design and innovation company, together with British medical start-up, Axis Spine Technologies, today announced a virtual reality (VR) solution which allows surgeons to evaluate and develop spinal implants virtually before making physical prototypes. Medical teams can collaborate more effectively, while also reducing costs and material waste.
Seymourpowell and Axis developed a modular system that surgeons can use to assemble a bespoke implant for each individual patient. The assembled implants consist of three customisable components, meaning a relatively small inventory of each can combine to create the ideal implant for any patient, compared to currently available technologies. Modularity gives surgeons choice to intra-operatively select the best implant for the patient.
VR was fully embraced during the implant design process. From kick-off, digital twins of the implant and instrumentation concepts were built. These were developed, evaluated and tested within a Seymourpowell-designed and coded VR platform.
Axis Spine Technologies Ltd., the emerging leader in modular interbody solutions, today announced completion of its Alpha release of the Axis-ALIF. This cage uses modular technology to reduce the damage to the vertebral endplates during cage insertion. Cage subsidence is a common complication which leads to poor clinical outcomes, lower fusion rates and increases the risks of revision surgery.
The Axis-ALIF is the company’s 1st product cleared for use in the United States. Surgeries have taken place in Atlanta GA, Raleigh NC and Dallas TX.

The presentation “Can a novel, modular ALIF cage help reduce loss of lordosis and provide 3D spinal alignment?” was very well received with several surgeons enquiring about the technology after the talk. Axis is committed to proving the benefits of the insertion methodology with both biomechanical and clinical studies.
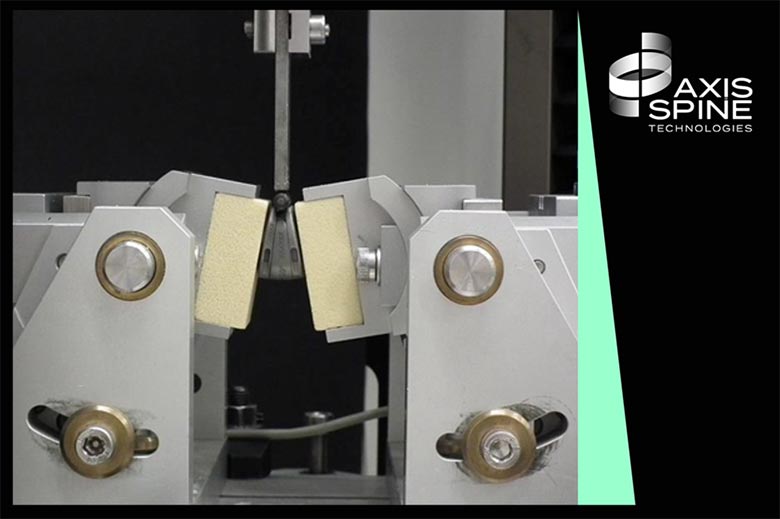
SpineServ (Ulm, Germany) has led a first in a series of biomechanical tests comparing the modular concept of The Axis-ALIF to the established 1-piece cage designs.
Under the auspice of Prof. Dr. Hans-Joachim Wilke, one of the world’s leading experts in spine biomechanics, the resistance to implant expulsion was compared between a modular cage and a 1-piece cage.
The hypothesis being that due to its modular insertion technique, the Axis-ALIF cage would cause less structural damage to the endplates upon insertion, and as such see an increased push-out resistance when compared to 1-piece cages.
The study results are in the process of submission for publication in a peer-reviewed medical journal.

Axis Spine Technologies (AST), a UK-based start-up with U.S. subsidiary that focuses on development and commercialisation of next generation spinal implants, announces its first U.S. Commercial Leadership appointment. Gant Newsom joins the organization as Vice President of U.S. Market Development. As such, Gant will be leading the company’s early U.S. commercialisation efforts.
With Gant AST onboards a wealth of Med Tech experience where he has held multiple roles from sales rep to sales executive. Gant has mainly been focusing on spine during the past 13 years with highly successful runs at DuraStat, Camber Spine, LDR/Zimmer Biomet and Globus Medical.

Axis Spine Technologies (AST) today announces it has received FDA 510(k) clearance for standalone use + extended applications of its differentiating modular ALIF device.
AST is the first company to now offer up to 40 degrees lordotic cages, as well as up to 20 degrees of coronally tapered endplates, allowing surgeons a range of correction capabilities far in excess of any other interbody implant, when treating lumbar degenerative and deformity patients.
Axis-ALIF is currently available for sale in the United States.
Spinal implant startup is the first to receive funding from ACF Investors’ Delta Fund, thanks to the backing of expert medtech angel investor
27, January 2021 – Axis Spine Technologies Ltd (AST) has raised £2.2m in a funding round led by ACF Investors, with follow-on investment from Mercia’s EIS funds. Mercia was the first investor when Axis was founded by Jon Acros in 2017. This is the first investment from ACF Investors’ new Delta Fund, a fast-track investment fund designed for high-potential UK businesses that have been backed by a syndicate of angel investors with deep sector knowledge. Axis is developing the next generation of Anterior Spinal Implant technology which provides surgeons with increased correction and alignment options. Its modular devices are inserted with less force than conventional implants to reduce the risks of structural damage which can lead to subsidence, an under-reported and recognised complication. read more…
Advances in interbody fusion implants are overshadowing the PSO-driven approach to spinal correction.